 Frederick, MD - SafetyTech International, the leading manufacturer of Powered Air Purifying Respirators (PAPRs) to the U.S. Military announces the introduction of the PureAir Bio Defense Kit ™Designed to protect users from biological contamination, the kit is NIOSH approved and iseal for First Receivers, Clean Rooms, Infectious Disease Labs and Hospital Staff receiving potentially contaminated patients. The PureAir Bio Defense Kit™ is NIOSH approved with (2) HE particulate filters. Worn on the waist, the
Frederick, MD - SafetyTech International, the leading manufacturer of Powered Air Purifying Respirators (PAPRs) to the U.S. Military announces the introduction of the PureAir Bio Defense Kit ™Designed to protect users from biological contamination, the kit is NIOSH approved and iseal for First Receivers, Clean Rooms, Infectious Disease Labs and Hospital Staff receiving potentially contaminated patients. The PureAir Bio Defense Kit™ is NIOSH approved with (2) HE particulate filters. Worn on the waist, the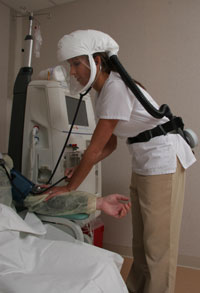 unit is compact, lightweight and operates on NiMH rechargeable, 8 hour disposable or (4) D-Cell batteries that run for greater than 12 hours. The complete system includes Loose Fitting Head Cover.The system does not require fit-testing and can be worn by users with facial hair. The loose fitting head cover permits easy use of a stethoscope. SafetyTech International, Inc., A subsidiary of TVI Corporation has fielded over 50,000 PAPR systems worldwide. The company has recently sold the PureAir Bio Response Kit™ in Asia where hospital staffs are dealing with the threat of an Avian Flu pandemic. For More Information Contact: Dennis Ottey SafetyTech International, Inc. Toll Free: 888-744-6462 or E-Mail: dottey@safetytechint.com www.safetytechint.com
unit is compact, lightweight and operates on NiMH rechargeable, 8 hour disposable or (4) D-Cell batteries that run for greater than 12 hours. The complete system includes Loose Fitting Head Cover.The system does not require fit-testing and can be worn by users with facial hair. The loose fitting head cover permits easy use of a stethoscope. SafetyTech International, Inc., A subsidiary of TVI Corporation has fielded over 50,000 PAPR systems worldwide. The company has recently sold the PureAir Bio Response Kit™ in Asia where hospital staffs are dealing with the threat of an Avian Flu pandemic. For More Information Contact: Dennis Ottey SafetyTech International, Inc. Toll Free: 888-744-6462 or E-Mail: dottey@safetytechint.com www.safetytechint.com
Subscribe to the DPJ Weekly Brief newsletter:
Subscribe
